How molecular relics in your cells tell the story of our common origins.
By Rich Feldenberg
In “Emma”, Jane Austin’s classic Novel, Emma Woodworth is described as handsome, clever, and rich. She takes to matchmaking, perhaps overestimating her abilities, and in doing so a variety of humorous and near disastrous calamities ensue. Of course, all ends well for Emma and her friends in the Novel. In this article we will examine a different sort of EMMA, but there may be some analogy to be found that even the brilliant Ms. Austin could not have foreseen. EMMAs is my acronym for Evolutionarily Modified Molecular Artifacts. I have used it in place of what has previously been referred to by some as molecular fossils. Fossil has the implication of something long dead, now extinct, and not seen in the world for many ages. Besides not being precisely what is meant by molecular fossil, when used by molecular biologists or astrobiologists, molecular fossil already has another meaning when referring to molecular or chemical remnants of past life. EMMAs may be a more appropriate term since it refers to molecular parts of still living systems that still display some resemblance to their more ancient and primitive forms. In this article we’ll explore a few examples of EMMAs and see what they can tell us about our distant past and the origin of life on earth. Austin’s Emma says “seldom, very seldom does complete truth belong to any human disclosure; seldom can it happen that something is not a little disguised or a little mistaken”. It is the nature of Evolutionarily Modified Molecular Artifacts, that their true nature is more than a little disguised and has traditionally been more than just a little mistaken. Lets look at the evidence that these living artifacts may give us a glimpse at a truth about our distant past, where we came from, and our common origins with our fellow living inhabitants on planet earth.
There are a number of critical biological molecules that are common to all life forms on earth today, and that have some unusual properties suggesting a common origin arising from more primitive precursor molecules. With this in mind, we’ll look at the common molecule ATP and the coenzymes NAD, and Acetyl Coenzyme-A, and finally the catalytic site of the protein synthesizing ribosome, which is perhaps the most fundamental molecular machine of any living cell. We’ll see that these examples also hint at a previous and now lost stage of life known as the RNA world, that preceded the Last Universal Common Ancestor (LUCA) of all living things on our planet today. To continue to stretch our Jane Austin analogy just a little further, we might imagine that the RNA world played matchmaker, in world long lost in deep time, and successfully paired DNA and protein, the two major biomolecules of life in our modern world. EMMAs demonstrate the remnants of that world before the matchmaking. Over evolutionary time they have been mesigned in their original forms, and re-mesigned into their current disguised forms. Like children who can not imagine a world before they were born, or before their parents existed, we too have a difficult time looking past the DNA/protein paradigm and into the RNA world.
Just like any good Austin Novel there are many interesting and complex characters. Some of the important players in our story of life on earth include molecules that contain pieces of ribonucleic acids (RNA). The first we’ll meet a key character known as adenine triphosphate (ATP). We will then be introduced to several of the coenzymes – small organic molecules that are necessary for the function of larger enzyme complexes. An finally we’ll become acquainted with one of the classic characters on life’s busy stage, the active site of the ribosome, which catalyzes one of the most fundamental reaction of the cell – the peptide bond to build protein. As stated above, each one of these molecules contains an RNA component, even though none of them are used to store or transfer genetic information. They are all involved in important biochemical reactions that have traditionally been thought to be performed only by protein enzymes. As we will see, the catalytic site of the ribosome relies on RNA exclusively to catalyze it’s fundamental reaction, and is therefore a ribozyme (RNA enzyme). These examples, and many others that we won’t describe today, appear to provide evidence of a long lost RNA world, with protein eventually evolving around the RNA core to assist and improve its biochemical efficiency.
First let’s look at the simple ATP molecule, which is well known to serve as the energy currency of the cell. It functions to power chemical reactions by transferring energy from its high energy phosphate bonds. It contains the base adenine, bound to the pentose sugar ribose. Ribose is the same sugar used in RNA (ribonucleic acid). The sugar ribose differs from the sugar deoxyribose (the sugar of DNA) only in the presence of a hydroxyl (OH) group at the 2-prime carbon. DNA does not contain this 2-prime hydroxyl group.
ATP is produced by the metabolic processes of glycolysis, the Kreb’s cycle, oxidative respiration, and by light powered photosynthesis, but is used in a multitude of reactions to provide the energy necessary to drive those reactions in the desired direction. Why should it be necessary that this energy storage molecule is a nucleotide? Could this be a hint that it’s important role began at a time when RNA played a much more central role in biology than it does today? Is the adenine now just a left over of the original mesign?

ATP – the energy currency of the cell.
Let me now introduce you to the charming NAD. Nicotinamide Adenine Dinucleotide(NAD) is a coenzyme that is composed of two ribose containing nucleotides linked together by a diphosphate connector. One of the nucleotides is adenine, just like that found in RNA, and the other nucleotide contains the non-RNA base nicotinamide. Being a dinucleotide, again should make us appreciate this coenzyme’s primitive origins.

Nicotinamide Adenine Dinucleotide (NAD)
The Adenine base is on the bottom half and the Nicotinamide is on the top half.
Nicotinamide is converted from nicotinic acid to its amide form. Nicotinic acid is also known as the vitamin niacin. The name was changed to niacin due the concern that people would confuse the nicotinic acid with nicotine and falsely believe that nicotine had nutritional health benefits. Nicotinic acid and nicotine are chemically distinct molecules, although they both share a pyridine ring structure- which is an aromatic heterocyclic ring with nitrogen at position 1 (see below). Both nicotinic acid and nicotine have their own distinct biological effects. Of course, nicotine is produced by the tobacco plant, but is not produced by animal cells. Nicotinic acid is found in all living cells, whether they are animal, plant, or single celled bacteria.



Chemical similarities between Nicotinamide (part of NAD) on the left, Nicotinic acid (Niacin) in the middle, and Nicotine (harmful carcinogen) on the right.
Nicotinamide Adenine Dinucleotide (NAD) is necessary for the operations of a wide variety of enzymes in all cells. The NAD molecule can be in either an oxidized form (NAD+) or a reduced form (NADH), and is therefore an important component of many oxidation-reduction reactions in the cell. It can transport electrons in its NADH form, or take them away in its NAD+ form. Since cell metabolism is, in large part, the process of extracting energy from biomolecules like sugars and fatty acids – in other words oxidizing these molecules in a slow and controlled way – NAD is important for the function of many enzymes found along these these metabolic pathways in the cytosol and mitochondria in eukaryotic cells. In the mitochondria NADH becomes oxidized, as electrons flow down the electron transport chain. The resulting H+ (proton) is pumped across the cellular membrane, creating a proton electrochemical gradient, which then is used to produce ATP – to be used to power other non-spontaneously occurring chemical reactions.

Ball and Stick chemical model of NAD
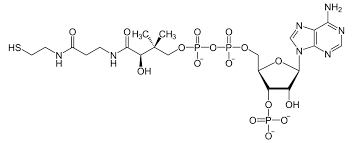
The coenzyme known as Acetyl-CoenzymeA , like NAD, also contains the nucleotide adenine. Connected to it is a molecule with a thiol group (SH) at its end. This molecule participates in important chemical reactions that require the transfer of an acetyl group (a methyl bonded to a carbonyl – see below).
Many steps in key chemical pathways involve acetyl transfers to build or break down molecules. The sulfur group in Coenzyme A can chemically attack the acetyl group of another molecule, remove it from that molecule, and thereby take it for use in a multitude of biochemical reactions. In the process Coenzyme A becomes Acetyl Coenzyme A, and can be recycled back to Coenzyme A once it released the acetyl group at the right time and place. It is an important part of enzymes involved in glycolysis and the Krebs cycle – both chains of reactions that break down glucose to create ATP. Gene expression can also be regulated by acetylation of histone protein, telling the cell which genes to transcribe and which need to remain silent in a given cell type. It is also used to create the neurotransmitter acetyl-choline from choline.
Conenzyme A. To become Acetyl-Coenzyme A, an acetyl functional group is attached to the thiol group at the far left end of the molecule. In this way, Acetyl-Coenzyme A can transport a carbon atom to be used in other chemical reactions.
Our true hero is the ribosome, the site of protein synthesis, and common to all modern cell types, although, the molecular structure differs enough between prokaryotes (single celled organisms like bacteria and archaea) and eukaryotes (more sophisticated cell types like that seen in animal or plant cells) that these differences can be exploited by certain antibiotics which target prokaryotic ribosomes, but leave the eukaryotic ribosomes unharmed. Even the mitochondria found in animal and plant cells have their own ribosomes that resemble prokaryotic ribosomes more than eukaryotic ribosome found in the cytoplasm of those same cells. The production of protein is perhaps the most primitive and basic metabolic function of all living cells. It came as a huge surprise to scientists when they learned that the active site of the ribosome (where the peptide bonding reaction takes place – the peptidyl transferase reaction) is composed only of RNA and no protein at all. Additional structural studies have confirmed that it is the RNA that catalyzes this basic cell reaction.
This would seem to support the notion that RNA played the major role in the biochemistry of the most primitive life forms. Ribosomes today are complex molecules, made of multiple components, some of which are ribosomal RNA and other parts are protein – it is therefore a ribonucleoprotein. The protein portions seem to assist the ribosome in doing its job more efficiently.
The examples given above reveal the important role that RNA molecules play in cellular biochemistry. The fact that some of the basic process of life rely on these RNA containing molecules lends support for the RNA world hypothesis. Except for the ribosome where the actual catalytic site is still a ribozyme, the other examples don’t use the RNA portion for the vital catalytic role, but may possibly have done so in the distant past. The presence of the RNA still retained in the coenzyme may offer proof that it is a molecular fossil – or as I prefer an Evolutionarily Modified Molecular Artifact (EMMA). To paraphrase Jane Austin, when referring to the RNA that lays hidden at the core of many of our most rudimentary metabolic processes, which may have served a grander role in a far distant past, and which now has relinquished it’s primary role for one of a more modest, behind the scenes assistant, “The sweetest and best of all molecules, faultless in spite of all her faults”. In future articles we can examine some other examples of EMMAs, such as additional types or ribozymes and riboswitches.
References:
1. Article on Mesign in Nature (also linked to within this article):
http://darwinskidneys-science.com/2015/07/08/another-clever-mesign-brought-to-you-by-mother-nature/
2. Nicotinamide Adenine Dinucleotide (NAD) Wiki article.
https://en.m.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide
3. Acetyl Coenzyme A Wikipedia article.
https://en.m.wikipedia.org/wiki/Acetyl-CoA
4. “The RNA World” Gesteland, Cech, and Atkins. Second Edition, Cold Spring Harbor Laboratory Press. 1999.
5. “Molecular Biology of the Gene” Watson, Hopkins, Roberts, Steitz, Weiner. Fourth Edition, 1987.
6. Life as we don’t know it. “Musings on the Biochemistry of Saturn’s Moon Titan”.