by Rich Feldenberg:
In our last Darwin’s Kidneys post we discussed the basic concept behind the second law of thermodynamics, which requires that entropy increase for every irreversible process. Entropy can be thought of as the amount of disorder in a system, so this law is essentially saying that there is an increase in the total amount of disorder that accompanies every physical process. We discussed why this law – which is thought to always hold true throughout time and space – does not prohibit the development of complex structure or the evolution of life, but it might also be true that the second law is a driving force behind the evolution of complexity in both living and non-living systems.
In this article I would like to continue our thermodynamic discussion, but introducing an interesting, although somewhat unproven and controversial offshoot of this scientific principle, which attempts to show that self organization of atoms and molecules is actually a consequence of second law dynamics. It’s founder and major proponent is a young physics professor at MIT, named Jeremy England. He has been attempting to show through a rigorous mathematical approach, that complexity arises naturally in physical systems as these systems move towards more efficient mechanisms to disperse energy – increase disorder in their surroundings. These systems become more efficient at increasing universal disorder, by becoming themselves more ordered. This work has potentially broad implications helping us understand how living systems might have arisen naturally from non-living systems, even before those systems were self-replicating and capable of Darwinian evolution.
The entropy of a closed system will always increase over time, but an open system allows an influx of energy so that the entropy of part of that system can decrease as the entropy of it’s surroundings increases. The geochemical environment of the early earth could be considered an open system because there was intense energy continuously entering into the system from the sun. Plants are extremely efficient at using that energetic sunlight to maximize the disorder of their surroundings. This is somewhat like looking at the problem upside down from our usual way of thinking. We normally think of plants evolving to use sunlight more effectively to become more complex, and as a natural consequence they create a larger entropy to the environment. England’s way of looking at the plant might be to say that second law demands that entropy will increase with time and the highly energetic sunlight will affect the system so that complexity will arise that will move towards maximum entropy generation. Those more effective entropy generators will necessarily be more complex systems, tending toward self-assembly and reproduction, and in some cases, eventually what we would recognize as living things. Living systems are very good at dissipating its energy.
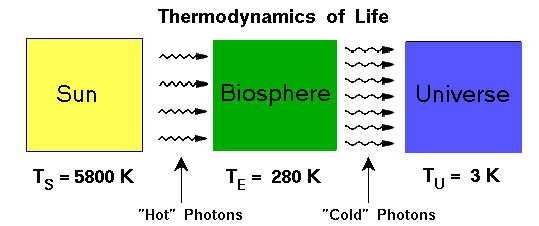
For these kind of processes to occur a system has to be out of thermodynamic equilibrium. At equilibrium there is no net energy transfer, but a system out of equilibrium has a net movement of energy – the influx of sunlight, for example. At some distant time in the future, the entropy of the entire universe will be high (the universe being a closed system), and at that point all areas of the universe will be in thermodynamic equilibrium, and complexity, organization, and life will cease to exist. Fortunately, it is likely to be a very long time before that fate befalls our universe.
England’s thermodynamic dissipative process might explain organized non-living structures we see everywhere in the world, from the formation of snowflakes and sand dunes, to planetary rings and spiral galaxies. These structures preferentially form to better disperse energy into more disordered and less usable forms – a consequence of thermodynamic’s second law. In this way, life itself is just one form of a more broad variation on this theme. Self organizing structures may have formed to raise entropy maximally, and in doing so lead to the first self-replicators. Once you have replicators, a Darwinian evolution by natural selection can take over to increase complexity further.
Not all researchers believe that Dr. England’s theory will pan out as a solution to the origin of life, but it seems that there are more than a few that have been impressed with the theory and its results so far. I have read two of England’s original journal articles, and unfortunately that math of the statistical mechanics was beyond me. From what other researchers have said, however, the equations used are valid, it is their interpretation for self assembly and origins of life, that is still unclear.
Professor England is himself and interesting individual. In his early 30s and approaching the origin of life field from a fresh perspective, England earned his PhD in physics at Stanford University in 2009, and is now an Assistant Professor of Physics at the Massachusetts Institute of Technology with his own research lab. In 2011 he was named as “one of the 30 under 30 rising stars in science”, by Forbes magazine. One thing that I found particularly fascinating is that although England is attempting to crack the tough nut of the origins of life, using sound science and mathematical modeling, he is a devout Orthodox Jew. He speaks somewhat to his faith and how he reconciles faith with his naturalistic scientific approach to answer this basic fundamental question, of interest to both science and religion, in his podcast interview that I linked to below. Faith and high level scientific inquiry may be a good topic for another time.
*
I look forward to following Dr. England’s future work, and watching if others pick up on it and extend it further. If England is right, then far from The Second Law of Thermodynamics being a repressor of complexity, it may more accurately be a driving engine of the spontaneous production of organization and complex systems.
References:
1. “Statistical physics of self-replication”, Jeremy L. England; The Journal of Chemical Physics. 139, 121923 (2013).
2. “Dissipative adaptation in driven self-assembly”, J.L. England; Nat Nanotechnol. 10(11):919-23, Nov 4, 2015.
3. “The New Physics Theory of Life”. Quanta Magazine. January 22, 2014.
https://www.quantamagazine.org/20140122-a-new-physics-theory-of-life/
4. “Origins of Life: A Means to a Thermodynamically Favorable End?” Yale Scientific. July 1, 2014.
http://www.yalescientific.org/2014/07/origins-of-life-a-means-to-a-thermodynamically-favorable-end/
5. The 7th Avenue Project (Podcast). “Biophysicist Jeremy England: A New Theory of Life”. May 3, 2015.
http://7thavenueproject.com/post/118064180870/biophysicist-jeremy-england-new-theory-of-life
6. “How can we be so complex if the second law of thermodynamics is true?” Darwin’s Kidneys. Dec. 4, 2015.
http://darwinskidneys-science.com/2015/12/04/how-can-we-be-so-complex-if-the-second-law-of-thermodynamics-is-true/